Plant immunity: a century-long voyage of discovery
We need new strategies for controlling plant diseases
Plant health has always been inextricably linked with human civilization: plant diseases have profound social and economic impacts, and changes in agricultural practices have likewise shaped the well-being of plants. One of the most famous historical examples is the blight that caused the Irish potato famine in 1845, resulting in one million people dying of starvation and roughly the same number being forced to emigrate. (Ireland’s population remains below 1845 levels now). Up to the present day, plant diseases have significant impact on the global economy: about 40% of crop yields worldwide are estimated to be lost to pests and diseases (Savary et al., 2019), which pushes up prices and threatens both livelihoods and food security. Moreover, losses that affect staples such as rice, wheat, maize, and potatoes are a major problem for ensuring food security and proper nutrition for humankind. To cite but four examples, potato losses due to late blight still result in annual crop losses that run to over 6 billion USD. Panama disease is destined to reduce the availability of the ubiquitous Cavendish banana, which is grown as a monoculture in Central America (Ploetz, 2015). Around 2012, coffee rust, a fungal disease of coffee plants reduced yields by 16% and pushed many smallholder farmers into poverty (Boudrot et al., 2016). Further, stem rust, a devastating disease of wheat that had essentially been eradicated in major wheat-growing areas, has in the last ten years been spreading throughout the world again, in part due to climate change (Singh et al., 2015). Taken together, the different rusts that infect wheat are estimated to account for annual losses of this staple crop of almost 3 billion USD.
Plant diseases have profound social and economic impacts.
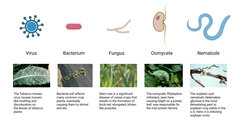
Plant pathogens are microorganisms that infect plants, causing disease. There are five major types of pathogens: viruses, bacteria, fungi, fungal-like oomycetes, and soilborne nematodes. Regardless of the pathogen type, their behaviors follow the same simple rules. They must find a susceptible host to infect, i.e., in which they can multiply, and then spread from host to host. The resulting losses have motivated farmers, researchers, and commercial plant breeders to devise strategies to reduce the percentage of crops that are lost to disease.
One major plank of this endeavor over the last 100 years has been the use of pesticides. A pesticide is defined by the EU Commission as an agent which “prevents, destroys, or controls a harmful organism (“pest”) or disease, or protects plants or plant products during production, storage and transport.” Pesticides have proven an effective strategy to control plant disease. They have contributed to global food security and were particularly important in conjunction with the growth of elite crops that were a product of the Green Revolution in the second half of the 20th century, which brought about huge increases in crop yields worldwide. Through the development of high-yielding dwarf wheat and rice varieties and improvement of agricultural practices, including the widespread use of chemical fertilizers, it is likely that Norman Borlaug, the “father of the Green Revolution”, contributed to saving millions of lives. However, while pesticides remain effective, their efficiency is ultimately limited, being essentially a way to manage rather than prevent plant disease. Further, their unbridled use is problematic due to adverse impacts on non-target organisms, including soil organisms even at sub-lethal doses (Gandara et al., 2024). For these reasons, the EU Commission has set the goal of halving pesticide applications in the EU by 2030 as part of its Farm to Fork strategy. Thus, while it is clear that chemicals will be required in some shape or form into the future, there is an increasing need for alternative crop protection strategies, especially those which can bring about resistance to disease rather than treating the symptoms.
While it is clear that pesticides will be required in some shape or form into the future, there is an increasing need for alternative crop protection strategies.
The journey that has taken us to this point – where we now have a molecular understanding of plant disease resistance – has proven to be a century-long voyage of discovery into the inner workings and complex relationships of plants and microbial pathogens. It shows us that progress in research is not linear but rather proceeds in bursts of knowledge. The journey also illustrates the importance of the continual development of new technologies and the courage of radical and innovative thinkers to question the prevailing scientific dogmas.
Plant breeding – first steps
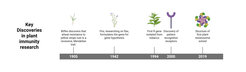
Resistance breeding, that is, the selection and development of disease-resistant plant lines, has been practiced by humans for millennia and began when plants were first domesticated about 10,000 years ago. Although there were notable success stories, for thousands of years, plant breeding in general was essentially, as described by English botanist John Lindley in the middle of the 19th century, “a game of chance played between man and plants”. Writing some years later, Charles Darwin lamented “the little which man had effected, by incessant efforts over thousands of years, in rendering the plants more productive or the grains more nutritious” (Evans, 1998), but the epochal findings of Gregor Mendel finally tilted the balance in the plant breeding game in favor of man. In Mendelian genetics, one version of a given trait is often dominant, and the other is recessive. Through making many crosses of his own – what we would today regard as classical plant breeding – the English botanist Rowland Biffen demonstrated that resistance to yellow stripe rust, a fungal disease of wheat, was a recessive Mendelian trait (Biffen, 1905). Although unappreciated at the time, the starting gun had been fired for efforts to harness insights into plant immunity to bolster disease resistance.
Plants and their pathogens: locked in a dance of life and death
The next key breakthrough in the field was made in the 1940s and 50s by Harold H. Flor, a flax breeder working at the United State Department of Agriculture station in North Dakota, where one of his key goals was to introduce resistance to flax rust disease. Flor discovered that disease resistance depended on an interaction that was determined equally by the genetic make-up of both the host and the pathogen (in this case a fungus). More specifically, based on his experiments in flax, Flor could show that both the inheritance of resistance in the host and the parasite’s ability to “cause resistance” are under the control of matching genes (Dodds, 2023). We now refer to the plant gene as a resistance (R) gene, whereas the pathogen’s “resistance-causing” gene is called an avirulence (Avr) gene. Plants that produce a specific R protein, often an intracellular protein with multiple domains, are resistant to a pathogen that produces the corresponding Avr protein. An equally important insight from Flor was that there is natural genetic diversity for the presence of R and Avr genes in both plant and pathogen populations, so that individuals in populations differ by the presence or absence, or variations, of these genes. The frequency of a given R and Avr gene in the host and pathogen population can change over time, which indicates that “disease” and “resistance” are dynamic rather than static traits at the population level. The R-Avr concept was formulated before it became clear what a gene is made of and what it encodes. A further remarkable conclusion of Flor’s so-called gene-for-gene hypothesis was that pathogens encode something that is getting them killed. Why would they want to do that?
This concept only came to fruition in the 1980s and 1990s when scientists began to isolate and identify first Avr and then R genes (Mansfield, 2009). By this time, it was well established that there was a dedicated recognition system in mammals, i.e., antibodies that detect diverse pathogens. Could the same be true for plants? It is worth mentioning that antibodies are part of the animal adaptive immune response which works on top of so-called innate immunity. By contrast, plants only possess innate immunity, meaning that all pathogen recognition capacity is "hard-wired" in the genes. Therefore, individual plant cells have to cope with pathogens without the mobile immune cells of vertebrates which constantly patrol the host organism for microbial invaders.
Plants only possess innate immunity, meaning that all pathogen recognition capacity is "hard-wired" in the genes. Therefore, individual plant cells have to cope with pathogens without the mobile immune cells of vertebrates.
Seismic technological advances played a key role in enabling discoveries, in particular, new techniques for molecular biology, such as “transposon tagging” and “chromosome walking”, that allowed the isolation and propagation (known as molecular cloning) of genes, for example in safe laboratory bacteria engineered for this purpose. With this method in hand, scientists could now isolate genes from pathogen strains that were resisted by plants and transfer them into strains that were not resisted. When the plants became resistant to the pathogen strain with a transferred gene, then researchers knew that they had successfully identified an Avr gene. The proteins encoded by these genes are now known as “effectors”, because their chief purpose is to “effect” infection on a suitable host plant which lacks a corresponding R gene.
Tracking down the corresponding R genes was facilitated by another significant development in plant biology research: the emergence of thale cress as a model system. Thale cress, or Arabidopsis thaliana, to give its scientific name, is a compact, rapidly growing flowering plant with a small genome – all qualities that make it tractable and suitable for research. One advantage of Arabidopsis was that it was easier for researchers to find R genes and subsequently transfer them into susceptible plant individuals (accessions) by generating transgenic Arabidopsis plants with the isolated R gene (see below). Another advantage was that this plant could also be used to study resistance to different classes of pathogens such as bacteria, viruses, fungi, and oomycetes in laboratory environments at relatively low cost. The first plant R genes isolated in the early 1990s and representing the two major receptor types for pathogen effectors were from Arabidopsis and tobacco (Bent et al., 1994; Mindrinos et al., 1994; Whitham et al., 1994).
New techniques enable new insights
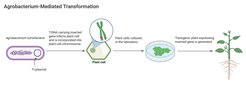
Characterizing the interacting genes in plants and pathogens responsible for resistance and infection was also made possible by significant breakthroughs in scientists’ ability to transfer foreign DNA into plant cells (Gelvin et al., 2003). Researchers made this discovery on the basis of investigations into bacteria called agrobacteria, which cause tumors in plants. It became clear that these tumors were caused by the agrobacteria transferring part of its genetic material into the genetic material of the plant cell. Could these bacteria be manipulated to transfer essentially any piece of DNA into plant cells? Indeed, scientists could demonstrate that the genes responsible for causing tumors could be removed without negatively impacting the ability of Agrobacterium to insert its DNA into the plant genome. This strategy was successfully used to produce the first transgenic plant – a tobacco plant expressing an antibiotic resistance gene (Herrera-Estrella et al., 1983) – and remains a key method to this day for plant scientists seeking to understand the functions of genes involved in infection and immunity.
Characterizing the interacting genes in plants and pathogens responsible for resistance and infection was made possible by significant technological breakthroughs.
In parallel to these efforts, other researchers were characterizing the microbial molecules that trigger activation of the plant immune system. From the late 1970s researchers knew that if they ground up the cell wall of a pathogen and applied it to plant cells this triggered a defensive response. Whatever was doing this had been termed an “elicitor” by scientists, but what exactly these elicitors were, remained unclear. Indeed, there were some hard-nosed geneticists who even rhetorically asked: “Wouldn’t any random chemical elicit a response if you threw it onto a plant cell?” It was mainly due to the tenacity with which Thomas Boller and Georg Felix countered this criticism that a much more interesting answer emerged: the discovery of membrane-resident pattern-recognition receptors (PRRs) on the surface of plant cells that perceive the presence of microbe-associated molecular patterns (MAMPs) made it clear that elicitor molecules often exhibit characteristic patterns, and, thus, the immune responses activated by these molecules are known as pattern-triggered immunity or PTI (Boller and Felix, 2009). Unlike the microbial molecules encoded by Avr genes, which tend to differ between individuals in a pathogen population, MAMPs are often conserved across a pathogen population. Membrane-anchored PRRs on the surface of plant cells survey the extracellular space between plant cells for the presence of MAMPs. Unlike R proteins that come in many types and forms, PRRs are often even conserved across an entire lineage of different species of flowering plants.
The discovery of membrane-resident pattern-recognition receptors (PRRs) on the surface of plant cells that perceive the presence of microbe-associated molecular patterns (MAMPs) made it clear that elicitor molecules often exhibit characteristic patterns.
Joining the dots

After a string of important discoveries at the end of the last century, the time was now ripe to conceptualize the plant immune system in its entirety. In 2006, two leading scholars of plant immunity formulated and codified some key principles and concepts (Jones and Dangl, 2006), in an attempt to better understand the different layers of plant immunity. Why, for example, do the proteins encoded by R genes intercept pathogen-derived AVR molecules inside plant cells? To answer this, it was necessary to take a step back and ask how the effectors work. It turned out that some of them block PTI, i.e., the immune signaling that is activated inside plant cells after recognition of MAMPs by PRRs on the plant cell surface. Putting this together, the authors conceptualized two layers of plant immunity that are activated in a back-and-forth between the pathogen and the plant that consists of four phases – a so-called “zig-zag model”.
Immune receptors revealed
While many R gene-encoded immune receptors were identified over the last 30 years, scientists had no clear idea of what these receptors look like and how they work. The last years have seen a burst of knowledge in this area, largely enabled by step changes in structural biology and biochemical techniques that have allowed researchers to visualize immune receptors at atomic resolution (Chai et al., 2023). With pioneering work from a structural biochemist, Jijie Chai, hitherto an outsider in this field, these breakthroughs have also synthesized and built on incremental knowledge accumulated by molecular geneticists from the last 25 years. One key principle that has emerged is the importance of R protein oligomerization. Plant immune receptors assemble into symmetrical, often strikingly beautiful, complexes known as resistosomes. Additional exciting insights have shown how these pathogen effector-activated resistosomes convert recognition into defense that protects plants against infection. One mechanism mediated by a widespread class of immune receptors involves the formation of small pores in the membranes of plant cells. These openings allow an influx of calcium ions, which then promote defense and often local plant cell death (the hypersensitive response), which stops an invading pathogen in its tracks. A second class of immune receptors catalyze the production of a suite of small molecules that activate downstream signaling components responsible for mediating disease resistance (Locci et al., 2023).
Looking ahead – harnessing plant immunity to safeguard food security
What do these fresh insights mean for plant immunity? After the two main classes of plant immune receptors had been identified, advances in cost-effective whole genome sequencing of complex crop genomes and those of their wild relatives revealed an enormous reservoir of R gene candidates. It is now clear that for over a century, plant breeders have tapped only a tiny fraction of this immense natural resource for the introduction of R genes from wild relatives into modern crops. However, introducing R genes from wild relatives into crops through conventional crossings is a lengthy and labor-intensive strategy that often takes a decade. Moreover, breeders are playing catch-up with pathogens that shift their geographic distribution and/or mutate effectors so that they are no longer recognized by an elite breeding variety – an inevitable consequence of Flor’s insight that “disease” and “resistance” are dynamic traits at the population level. What’s more, only individuals of the same or very similar species can be crossbred, and potentially useful genes from more distant species remain out of reach. Now that R genes and their corresponding proteins are better understood, is there a better way to introduce resistance to existing valuable, high-yielding crops?
It is now clear that for over a century, plant breeders have tapped only a tiny fraction of the natural reservoir of resistance genes in wild relatives for introduction into modern crops.
Engineering and stacking – potentially effective strategies for exploiting the power of plant immune receptors
In recent years, scientists have started using cutting-edge gene editing technologies to engineer novel immune receptors that could, in principle, be made-to-order, based on in-depth knowledge of how these proteins work at a molecular level (for example, Cesari et al., 2022; Förderer et al., 2022). Much of this research has focused on immune receptors inside cells and on making these more active or with an expanded immune recognition. Designer monoclonal antibodies have already made a big splash in humans, and now that the operational “rules” are known, such receptors can also be designed in plants to intercept a particularly devastating plant pathogen strain by incorporating a small bespoke antibody recognition capability (Kourelis et al., 2023). Recent advances also mean that scientists can use artificial intelligence to design immune receptors with altered and/or expanded recognition specificities that render plants more resistant against a wider range of potential invaders. Another welcome consequence of new genome editing approaches is that scientists can more quickly move from, or in some cases completely bypass, traditional model organisms such as thale cress and directly work on crop species with real-world importance. One promising approach to engineering more resistant plants involves the “stacking” of R genes. This strategy involves the deploying of several R genes together that recognize multiple pathogen effector molecules in different ways and resulting in a longer-term resistance that is less likely to be overcome by evolving pathogen strains. In one example with clear real-world relevance, it has been demonstrated that using genome editing to introduce a combination of five resistance genes into wheat gives this crop robust resistance against stem rust, which is a major cause of losses in wheat yields, especially in Africa (Luo et al., 2021).

It is important to bear in mind that genome editing is not a universal solution for all plant breeding challenges. Indeed, it is most helpful to view it as a one – highly efficient and effective – tool in a box that also contains other useful tools. Challenges remain. For example, for some plant–pathogen interactions the AVRs matching known R genes have yet to be identified. Also, it is not straightforward to predict the pace of genetic adaptation in the pathogen when it faces simultaneous selection pressure from multiple R genes that are stacked. In addition, some plant pathogens which feed on dying plant cells are known as necrotrophs, and for these it is hard to find effective R genes, as encountered with “hard-to-crack” diseases such as Fusarium ear blight of wheat, a notorious fungus that appears to have found several alternative ways to cause disease. In purely scientific terms, however, genome editing approaches are often preferable over more traditional breeding strategies, owing to the relative ease, precision and shorter time frame they can be deployed in.
Genome editing is not a universal solution for all plant breeding challenges. It is most helpful to view it as a one – highly efficient and effective – tool in a box that also contains other useful tools.
Altering the DNA of plants – a thorny societal issue
The major stumbling block hampering introduction of genome editing remains societal acceptance of measures that involve changing the DNA of a plant. Further restrictions are imposed by the fact that a new gene-edited version of a plant must have approval for any territory to which it could conceivably gain access. Two aspects are worthy of particular mention in this regard. First, the – in many places – highly restrictive laws governing genetically modified organisms were made at a time when little was known about what gene editing could entail. What were – to quote former US secretary Donald Rumsfeld – the unknown unknowns? Owing to our enhanced understanding of cell and molecular biology as well as the availability of precision editing tools, scientists can now anticipate the effects of tailored interventions into the genomes of plants and other organisms. A second stumbling block for many appears to be the notion that by tinkering with the DNA of some of our staple crops to improve them, we are somehow ruining a perfect, primordial state. In fact, crops such as modern wheat are the product of extensive human breeding over millenia and have a high degree of genetic variation, which makes variation introduced by targeted gene editing very small in comparison (Jones, 2022). Additionally, if R genes are considered, then the vast repertoire of R genes in natural populations of non-domesticated plants has been unintentionally eroded through conventional plant breeding of elite varieties. Science communication will play a major role in future developments: if these concepts could be communicated more effectively, genetic modification of plants and its potential to increase food production may come to enjoy more widespread support.
If the science underpinning plant genome editing could be communicated more effectively, genetic modification of plants and its potential to increase food production may come to enjoy more widespread support.
Laws governing the modification of plants are becoming less restrictive
Indeed, it has become clear to decision-makers at national and international levels that innovation will be required if we are to meet the challenge of ensuring food security and protecting the environment in the years to come, broad ambitions which touch on many of the UN’s Sustainable Development Goals. Therefore, we need to use all the tools in the plant breeding toolbox. The EU Commission has proposed establishing a category of plants that should be exempted from GMO legislation because the modifications could, in theory, arise from natural mutation or conventional breeding approaches. Some of these proposed guidelines are very specific. For example, any insertions should not be longer than 20 nucleotides, as the likelihood of insertions of over 20 nucleotides occurring in large genomes – such as those of most crop species – is low. Under new rules, which are set to come into effect in the EU, the products of new gene-editing techniques like the highly efficient CRISPR-Cas9 gene editing tool will be exempt from the most strict risk assessment and labelling requirements, making it much easier to use these tools to produce and protect our food. In the UK, the government signed into law the Genetic Technology (Precision Breeding) Act in 2023 which is less focused on the technology and more oriented to the outcome. That is, if, for example, a given gene could feasibly be incorporated into a plant by traditional breeding, then its incorporation using a gene editing technology would also be deemed safe.
To meet ambitious environmental goals and ensure food security, we will need to use all the tools in the plant breeding toolbox.
One notable beneficiary of this new flexibility might be our good friend, the potato. Potato breeding has traditionally been very difficult. This is because instead of inheriting one copy of every chromosome from both the father and from the mother plant (as in humans), potatoes inherit two copies of each chromosome from each parent, making them a species with four copies of each chromosome (tetraploid). Four copies of each chromosome also mean four copies of each gene, and this makes it highly challenging and time-consuming to generate new varieties that harbor a desired combination of individual properties. New genome technology developments now mean that we may be able to protect potato crops from disease to a much greater extent than has been previously possible. In England, potatoes are now being tested that harbor genes providing resistance against blight as well as viral infection of seeds. Crucially, these genes originate from other wild potato accessions, meaning that they would meet the stipulations of the UK’s Precision Breeding Act.
Pesticides are now one strategy among many to protect plants.
Coming back to the use of pesticides discussed at the outset, these agents will likely still be required for the foreseeable future to ensure that enough food is produced to feed the world. At the same time, insights into how plant immunity works at a molecular level – and therefore precise molecular changes that could make immunity more effective – mean that scientists have identified an array of processes and pathways that can be targeted to better protect crops from disease. Such a strategy can be used alongside judicious and limited use of chemical fertilizers. Indeed, pharmaceutical companies such as Bayer have started to phase out some pesticides and to provide alternatives. Some companies have shifted their focus to so-called “biologicals”, essentially anything that has a natural source. One example is the fungicide Serenade®, which is based on the bacterium Bacillus subtilis, and whose mode of action is purported to involve direct toxicity to pathogenic fungi and induction of plant immunity. However, caution is required around the use of bacterial inoculants in agriculture, as some recent analyses have revealed that, in many cases, such inoculants do not exert robust positive effects (Giller et al., 2024; Koziol et al., 2024).
Conclusion – we’ve come a long way in 100 years.
Our understanding of plant immunity has come a long way since the turn of the 20th century. From not knowing what genes were, we now, in some cases, have a detailed understanding of how the products of plant R genes interact with pathogens and set in motion immune signaling leading to effective disease resistance. From breeding being “a game of chance”, we now have precise tools that allow scientists to transfer genes between organisms, to turn genes on or off, and make exquisite alterations to their coding sequences to improve pathogen recognition. While exciting developments and discoveries will continue to be made, our current challenges are not only harnessing new scientific insights but also ethics and societal acceptance: how do we want to use the tools that we now have at our disposal?
Acevedo-Garcia J, Kusch S, Panstruga R. Magical mystery tour: MLO proteins in plant immunity and beyond. New Phytol. 2014 Oct;204(2):273-81. doi: 10.1111/nph.12889. PMID: 25453131.
Bent AF, Kunkel BN, Dahlbeck D, Brown KL, Schmidt R, Giraudat J, Leung J, Staskawicz BJ. RPS2 of Arabidopsis thaliana: a leucine-rich repeat class of plant disease resistance genes. Science. 1994 Sep 23;265(5180):1856-60. doi: 10.1126/science.8091210. PMID: 8091210.
Biffen RH. Mendel’s law of inheritance and wheat breeding. J Agric Sci. 1905. 1;4–48
Boller T, Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379-406. doi: 10.1146/annurev.arplant.57.032905.105346. PMID: 19400727.
Boudrot A, Pico J, Merle I, Granados E, Vílchez S, Tixier P, Filho Ede M, Casanoves F, Tapia A, Allinne C, Rice RA, Avelino J. Shade Effects on the Dispersal of Airborne Hemileia vastatrix Uredospores. Phytopathology. 2016 Jun;106(6):572-80. doi: 10.1094/PHYTO-02-15-0058-R. Epub 2016 Apr 4. PMID: 26828230.
Butelli E, Titta L, Giorgio M, Mock HP, Matros A, Peterek S, Schijlen EG, Hall RD, Bovy AG, Luo J, Martin C. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat Biotechnol. 2008 Nov;26(11):1301-8. doi: 10.1038/nbt.1506. Epub 2008 Oct 26. PMID: 18953354.
Büschges R, Hollricher K, Panstruga R, Simons G, Wolter M, Frijters A, van Daelen R, van der Lee T, Diergaarde P, Groenendijk J, Töpsch S, Vos P, Salamini F, Schulze-Lefert P. The barley Mlo gene: a novel control element of plant pathogen resistance. Cell. 1997 Mar 7;88(5):695-705. doi: 10.1016/s0092-8674(00)81912-1. PMID: 9054509.
Cesari S, Xi Y, Declerck N, Chalvon V, Mammri L, Pugnière M, Henriquet C, de Guillen K, Chochois V, Padilla A, Kroj T. New recognition specificity in a plant immune receptor by molecular engineering of its integrated domain. Nat Commun. 2022 Mar 21;13(1):1524. doi: 10.1038/s41467-022-29196-6. PMID: 35314704; PMCID: PMC8938504.
Chai J, Song W, Parker JE. New Biochemical Principles for NLR Immunity in Plants. Mol Plant Microbe Interact. 2023 Aug;36(8):468-475. doi: 10.1094/MPMI-05-23-0073-HH. Epub 2023 Sep 11. PMID: 37697447.
Dodds PN. From Gene-for-Gene to Resistosomes: Flor's Enduring Legacy. Mol Plant Microbe Interact. 2023 Aug;36(8):461-467. doi: 10.1094/MPMI-06-23-0081-HH. Epub 2023 Sep 11. PMID: 37697270.
Evans, LT. Feeding the Ten Billion: Plants and population growth. Cambridge University Press, Cambridge. 1998
Förderer A, Li E, Lawson AW, Deng YN, Sun Y, Logemann E, Zhang X, Wen J, Han Z, Chang J, Chen Y, Schulze-Lefert P, Chai J. A wheat resistosome defines common principles of immune receptor channels. Nature. 2022 Oct;610(7932):532-539. doi: 10.1038/s41586-022-05231-w. Epub 2022 Sep 26. PMID: 36163289; PMCID: PMC9581773.
Gandara L, Jacoby R, Laurent F, Spatuzzi M, Vlachopoulos N, Borst NO, Ekmen G, Potel CM, Garrido-Rodriguez M, Böhmert AL, Misunou N, Bartmanski BJ, Li XC, Kutra D, Hériché JK, Tischer C, Zimmermann-Kogadeeva M, Ingham VA, Savitski MM, Masson JB, Zimmermann M, Crocker J. Pervasive sublethal effects of agrochemicals on insects at environmentally relevant concentrations. Science. 2024 Oct 25;386(6720):446-453. doi: 10.1126/science.ado0251. Epub 2024 Oct 24. PMID: 39446951.
Gelvin SB. Agrobacterium-mediated plant transformation: the biology behind the "gene-jockeying" tool. Microbiol Mol Biol Rev. 2003 Mar;67(1):16-37, table of contents. doi: 10.1128/MMBR.67.1.16-37.2003. PMID: 12626681; PMCID: PMC150518.
Giller KE, Ardley J, James EK, Unkovich MJ. Science losing its way: examples from the realm of microbial N2‑fixation in cereals and other non‑legumes Plant Soil. 2024 https://doi.org/10.1007/s11104-024-07001-1
Herrera-Estrella L, Depicker A, Van Montagu M, Schell J. Expression of chimaeric genes transferred into plant cells using a Ti-plasmid-derived vector. 1983. Biotechnology. 1992;24:377-81. PMID: 1422044.
Jones JD. Curr Biol. 2022 Dec 5; R1292-R1293. doi: 10.1016/j.cub.2022.10.035
Jones JD, Dangl JL. The plant immune system. Nature. 2006 Nov 16;444(7117):323-9. doi: 10.1038/nature05286. PMID: 17108957.
Kourelis J, Marchal C, Posbeyikian A, Harant A, Kamoun S. NLR immune receptor-nanobody fusions confer plant disease resistance. Science. 2023 Mar 3;379(6635):934-939. doi: 10.1126/science.abn4116. Epub 2023 Mar 2. PMID: 36862785.
Koziol L, McKenna TP, Bever JD. Meta-analysis reveals globally sourced commercial mycorrhizal inoculants fall short. New Phytol. 2025 May;246(3):821-827. doi: 10.1111/nph.20278. Epub 2024 Nov 21. PMID: 39569734.
Locci F, Wang J, Parker JE. TIR-domain enzymatic activities at the heart of plant immunity. Curr Opin Plant Biol. 2023 Aug;74:102373. doi: 10.1016/j.pbi.2023.102373. Epub 2023 May 5. PMID: 37150050.
Luo M, Xie L, Chakraborty S, Wang A, Matny O, Jugovich M, Kolmer JA, Richardson T, Bhatt D, Hoque M, Patpour M, Sørensen C, Ortiz D, Dodds P, Steuernagel B, Wulff BBH, Upadhyaya NM, Mago R, Periyannan S, Lagudah E, Freedman R, Lynne Reuber T, Steffenson BJ, Ayliffe M. A five-transgene cassette confers broad-spectrum resistance to a fungal rust pathogen in wheat. Nat Biotechnol. 2021 May;39(5):561-566. doi: 10.1038/s41587-020-00770-x. Epub 2021 Jan 4. PMID: 33398152.
Mansfield JW. From bacterial avirulence genes to effector functions via the hrp delivery system: an overview of 25 years of progress in our understanding of plant innate immunity. Mol Plant Pathol. 2009 Nov;10(6):721-34. doi: 10.1111/j.1364-3703.2009.00576.x. PMID: 19849780; PMCID: PMC6640528.
Mindrinos M, Katagiri F, Yu GL, Ausubel FM. The A. thaliana disease resistance gene RPS2 encodes a protein containing a nucleotide-binding site and leucine-rich repeats. Cell. 1994 Sep 23;78(6):1089-99. doi: 10.1016/0092-8674(94)90282-8. PMID: 7923358.
Ploetz RC. Fusarium Wilt of Banana. Phytopathology. 2015 Dec;105(12):1512-21. doi: 10.1094/PHYTO-04-15-0101-RVW. Epub 2015 Nov 23. PMID: 26057187.
Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A. The global burden of pathogens and pests on major food crops. Nat Ecol Evol. 2019 Mar;3(3):430-439. doi: 10.1038/s41559-018-0793-y. Epub 2019 Feb 4. PMID: 30718852.
Singh RP, Hodson DP, Jin Y, Lagudah ES, Ayliffe MA, Bhavani S, Rouse MN, Pretorius ZA, Szabo LJ, Huerta-Espino J, Basnet BR, Lan C, Hovmøller MS. Emergence and Spread of New Races of Wheat Stem Rust Fungus: Continued Threat to Food Security and Prospects of Genetic Control. Phytopathology. 2015 Jul;105(7):872-84. doi: 10.1094/PHYTO-01-15-0030-FI. Epub 2015 Jun 29. PMID: 26120730.
Whitham S, Dinesh-Kumar SP, Choi D, Hehl R, Corr C, Baker B. The product of the tobacco mosaic virus resistance gene N: similarity to toll and the interleukin-1 receptor. Cell. 1994 Sep 23;78(6):1101-15. doi: 10.1016/0092-8674(94)90283-6. Erratum in: Cell 1995 May 5;81(3):466. PMID: 7923359.
Figure Panel of Plant Diseases
- Tobacco mosaic virus on tobacco - By R.J. Reynolds Tobacco Company Slide Set - USDA Forest Service, http://www.forestryimages.org/browse/detail.cfm?imgnum=1402027, Public Domain, https://commons.wikimedia.org/w/index.php?curid=12089941
- Bacterial wilt - by Eeshie - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=2940357
- Wheat stem rust - Public Domain, https://commons.wikimedia.org/w/index.php?curid=1214935
- Late blight on potato leaf – by Howard F. Schwartz, Colorado State University, United States - http://www.forestryimages.org/browse/detail.cfm?imgnum=5362902, CC BY 3.0, https://commons.wikimedia.org/w/index.php?curid=7933322
- Soybean cyst nematode - Public Domain, https://commons.wikimedia.org/w/index.php?curid=1214836
Figure Key discoveries in plant immunity research
created in https://BioRender.com
Figure Agrobacterium-mediated transformation
created in https://BioRender.com
FIGURE Zig-zag model
created in https://BioRender.com
FIGURE Field of wheat
Josep Monter via pixabay.com (whea-7347852 image)
Figure Crispr-Cas
created in https://BioRender.com




