Identity of an induced protein complex linking pathogen-activated immune receptors to rapid mobilization of immunity
Scientists from MPIPZ in collaboration with the Sainsbury Laboratory (UK) find a long sought after complex between two conserved plant-specific protein families that connect pathogen-activated immune complexes to defence outputs.
Most microbes are harmless to plants because receptor panels detect microbial disturbance outside and inside host cells to trigger innate immunity responses that block invasion. Receptors with a central nucleotide-binding domain (called NLRs) are intracellular molecular switches that become activated by infecting pathogens. The details of NLR activation and downstream signalling leading to defence are intensively researched, and they have important implications for global food security.
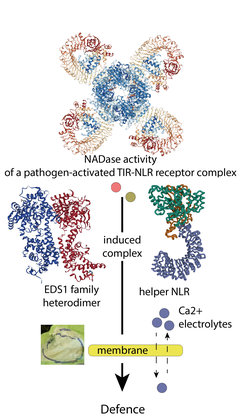
In dicot plants, such as Arabidopsis, lettuce and apple, NLRs contain an N-terminal signalling part which resembles Toll and interleukin-1 receptor (TIR) domains in animals. Plant TIR-NLRs can act as pathogen-activated NAD+ hydrolyzing oligomers and the NADase products are then thought to unleash immunity cascades which stop pathogen growth. Several additional proteins make this happen. These are evolutionary conserved “helper” NLRs and a family of three plant-specific lipase-like proteins EDS1, SAG101 and PAD4 (called the EDS1 family). Recent genetic studies formed the idea of a hard-wired coevolved functional network between certain helper NLR sub-types and EDS1 family members. How this network assembles at the biochemical level remained unclear.
To answer this question, Xinhua Sun and Dmitry Lapin from Parker laboratory teamed up with Joanna Feehan in the group of Jonathan Jones at the Sainsbury Laboratory in the UK. In collaboration with proteomics groups of Iris Finkemeier, Frank Menke, and Hirofumi Nakagami, they found that EDS1 family proteins associate with helper NLRs once a TIR-NLR receptors is specifically activated by pathogen-secreted proteins. An induced interaction between an EDS1 family heterodimer and the helper NLR occurs rapidly and transiently but is essential for effective immunity involving localized host cell death. By testing a derived helper NLR oligomer model and EDS1 family crystal structure information, the scientists identified key features required for assembling a functional immune signalling complex, working downstream of TIR-NLR activation and orchestrating anti-pathogen defences. One anticipated important early output mediated by the induced EDS1 family/helper NLR complex, based on recent work by colleagues, is calcium (Ca2+) and electrolyte movement across host cellular membranes, which stimulates defences for effective local and systemic immunity. The work described here provides a first biochemical underpinning for the initial transmission of pathogen sensing by TIR-NLR receptors to regulated execution of pathogen resistance.
[contributed by Dmitry Lapin and Jane Parker]
Read also the press release from The Sainsbury Laboratory, UK.
