Smallest suicide unit of immune response in plants identified
So-called "coil-coil domain" drives cells to programmed cell death
Plants do not surrender to pathogenic invaders without putting up a fight. They put up strong resistance with the weapons provided to them by their innate immune system. Paul Schulze-Lefert from the Max Planck Institute for Plant Breeding Research and his colleagues have stripped one of these tools down to its individual components and examined it in detail. The part of the protein that forces the infected cell to commit suicide in order to save the rest of the plant behaves like a portentously loaded spring. When detached from the protein, it sometimes delivers the deathblow to the cell without reason; but when embedded in the entire protein, it only does so in response to the corresponding signal.
The innate immune system of plants has sensors that register the presence of a microbial invader in the cell. Barley has an entire collection of such sensors whose names are composed of the letters MLA and an Arabic numeral. Each sensor consists of three domains which are attached one behind the other and have different functions. The most variant-rich domain recognises the different microbial enemies. A second strongly conserved domain alters the form of the sensor as soon as an intruder has been sighted. This change in form also acts as the starting signal for the activation of the third domain which is also strongly conserved. The latter delivers the deathblow to the cell by releasing the brake on the internal “suicide” programme.
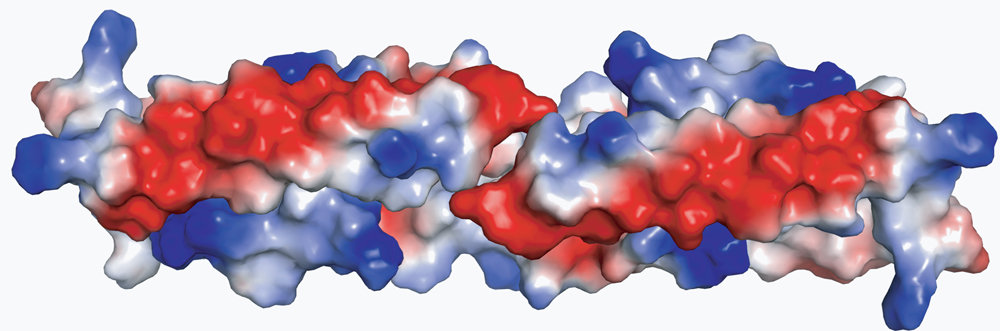
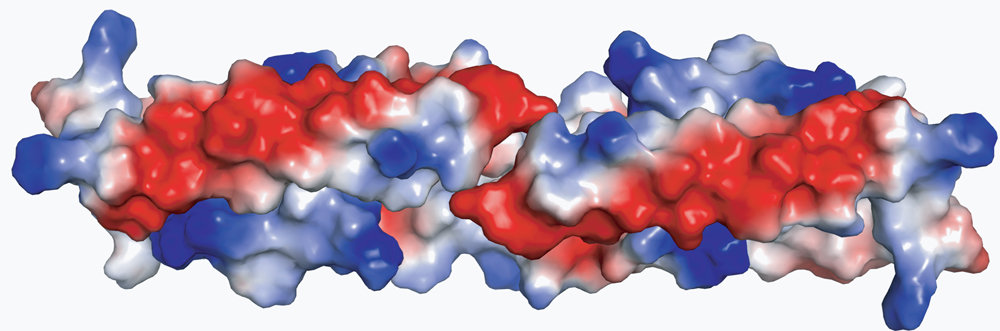
Paul Schulze Lefert and his colleagues focused specifically on the domain that drives the cell to its programmed cell death. It is referred to as the coil-coil domain. Schulze-Lefert and his team examined its structure and function in detail both when it is detached from the two other domains and when packed with them. The exact three-dimensional structure was determined in an x-ray diffraction analysis. The coil-coil domain is shaped like a long twisted screw with a short untwisted section in the middle, known as a helix-loop-helix structure. As the scientists write in the latest edition of the journal Cell Host&Microbe, two of these units form a homodimer. “The homodimer looks like two bent and interlocking wires that are wound in a spiral form except at the bending point. Thanks to the long parallel surfaces of the U-shaped ends, the structure is extremely stable and almost impossible to dismantle. However, if individual amino acids are modified, the homodimer becomes unstable and disintegrates,” explains Schulze-Lefert.
The international team working with Schulze-Lefert also succeeded in demonstrating that barley not only forms the homodimer in response to the intrusion of a pathogen, for example powdery mildew, it also does so without coming into contact with the enemy. The homodimer arises if the isolated domain is simply infiltrated into a plant cell. The coil-coil domain is also present in the double package in the complete sensor protein. The same applies for the two other domains. Thus, the entire sensor is also a dimer whose aggregation requires, however, the support of a few helper proteins.
The dimer loses its capacity to function as a result of the targeted modification of the coil-coil domain. Almost every mutation prevents the cell from being able to be forced to commit suicide. Therefore, strict requirements must apply regarding the correct structure. This also explains why this domain has hardly changed at all in the course of evolution. It is found in almost identical form in other plants. The plant sensors also display great similarity to the sensors found in animal innate immune systems, including that of humans. When integrated into the entire sensor, the coil-coil only drives the cell to its programmed death if it receives a corresponding signal. If released from the leash, it forces the cell to self-destruct, even if there is no signal. Schulze-Lefert explains: “The isolated domain is like a loose cannon ball. It alone is necessary and sufficient for the suicide of the plant cell. When integrated into the sensor, it behaves like a portentously loaded spring which must wait for its signal before it can become the angel of death.”
The issue of the journal Cell Host&Microbe in which Schulze-Lefert and his colleague’s work is published also contains a study by Peter N. Dodds from CSIRO Plant Industry in Canberra, Australia. Dodds and his group come to the same conclusion as the Cologne-based scientists: the only difference being that they used a different sensor from a different plant, namely flax. This demonstrates the general significance of the findings for the functioning of plant immune sensors.
CW/HR